2022/05/26
A Clinical Doctor's Explanation of Health Tech in Asia (Series#4)
Aevice Health Develops Wearable Stethoscopes for Chronic Respiratory Disease Patients
Medical communication, Clinical Doctor
The Usefulness of “AeviceMD” and Challenges for Clinical Use

Source: Aevice Health Pte Ltd
About Aevice Health
Aevice Health is a Singapore-based MedTech company from Nanyang Technological University. Adrian Ang, CEO and co-founder of Aevice Health has had asthma since childhood and has repeatedly been in and out of the hospital. This experience led him to establish a company to develop digital solutions to control chronic respiratory diseases such as asthma and chronic obstructive pulmonary disease (COPD). The product thus developed is “AeviceMD,” a smart wearable stethoscope for chronic respiratory disease patients to manage and monitor their symptoms at home. The company is a pre-series A company and has already raised US$3.2 million in funding and has partnerships with Toho Holdings, a Japanese listed company, and others.

Source: Aevice Health Pte Ltd
About “AeviceMD”
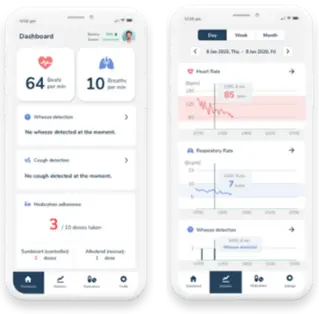
“AeviceMD” is a coin-sized wearable stethoscope (approximately 3cm in diameter) that provides patients with chronic respiratory disease a way to manage their condition and to stay connected to their clinicians while at home. The device is worn on the right side of the chest while the patient is resting or sleeping and continuously records heart rate and respiratory rate. It also constantly monitors breathing and heart sounds and detects and records wheezing (abnormal breathing sounds that occur during attacks of chronic respiratory diseases such as asthma).
The transmitted and recorded data can be shared with clinicians, who can then learn more about the severity of the disease and the effectiveness of treatment. Therefore, it is believed that these data can lead to better treatment outcomes of chronic respiratory diseases. “AeviceMD” is currently undergoing clinical trials in Singapore and is pending approval from the U.S. Food and Drug Administration (FDA). The company is also actively considering bringing the device to Japan.
Source: Aevice Health Pte Ltd
(Continue to the next)
The information contained in this article is compiled by the respective authors based on publicly available information. We assume no responsibility whatsoever for any damage or disadvantage caused by actions taken based on such information. Unauthorized reproduction of articles, photos, charts, etc. is prohibited.
Copyright © 2022 LSMIP office / CM Plus Corporation
A Series of Articles

